Abstract
Background
Oilseed plants of the Brassicaceae plant family are cultivated for food, feed and industrial purposes on large-scale in Europe. This review gives an overview of current market-oriented applications of new genomic techniques (NGTs) in relevant Brassicaceae oilseed crops based on a literature survey. In this respect, changes in oil quality, yield, growth and resistance to biotic and abiotic stress are under development in oilseed rape (Brassica napus), camelina (Camelina sativa), and pennycress (Thlaspi arvense).
Main findings
Environmental risk scenarios starting with hazard identification are developed for specific NGT applications in Brassicaceae oilseed crops with either a changed oil composition or with fitness-related traits. In case of a changed oil composition, an increase or decrease of polyunsaturated fatty acids (PUFA) may lead to risks for health and survival of pollinators. Regarding fitness-related traits, other risks were identified, i.e. an increased spread and persistence of NGT plants. Furthermore, there are indications for potential disturbance of interactions with the environment, involving signalling pathways and reaction to stress conditions.
Conclusion
It is shown that for environmental risk scenarios of the technological specificities of NGTs, the plants’ biology and the scale of releases have to be considered in combination. Therefore, the release of NGT plants into the environment for agricultural purposes will, also in future, require risk assessment and monitoring of individual traits as well as of combinatorial and long-term cumulative effects. In addition, risk management should develop concepts and measures to control and potentially limit the scale of releases. This is especially relevant for NGT Brassicaceae in Europe, which is a centre of diversity of this plant family.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Brassicaceae is a diverse plant family and includes many domesticated species (broccoli, cabbage, cauliflower, collards, kale, radish) as well as wild species, such as thale cress (Arabidopsis thaliana), capsella (Capsella bursa-pastoris), wild radish (Raphanus raphanistrum) and pennycress (Thlaspi arvense). The cultivated species of this plant family are global sources of vegetables, oil and seeds harvested to produce mustard. The oil is mainly obtained from the oilseed crops, i.e. oilseed rape (Brassica napus) and camelina/false flax (Camelina sativa) and can be used as source of food, for agrofuels, or for industrial purposes. Several species of this plant family originate from Europe and they partially emerged from interspecies hybridisation, such as Brassica napus [1,2,3]. Some of the recent species still have a potential for cross-hybridisation within the Brassicaceae plant family [4, 5].
Plant breeders have been working for many decades with Brassicaceae oilseed plants to improve both the quality of food and feed as well as plant-based raw material used in industry. So far, they have successfully altered traits such as the oil content and the fatty acid composition of the oilseed crops [6,7,8]. The oil obtained from Brassicaceae oilseed crops consists mainly of monounsaturated fatty acids or MUFAs (oleic, erucic and gondoic acids), polyunsaturated fatty acids or PUFAs (linoleic and linolenic acids) and saturated fatty acids (palmitic, stearic and eicosanoic acids) [9, 10]. Linoleic and linolenic acids are also known as omega-6 and omega-3 fatty acids, respectively. Both are known to have numerous health benefits [11], but are also known for their oxidative instability [12]. Depending on the intended final use, some studies have reported an increase (in food and feed, see [13]) or decrease (for food or industrial purposes, see [14,15,16]) in the PUFA content in Brassicaceae crops. In addition, attempts are being made to further reduce the erucic acid content. There is evidence that it is toxic for humans, however earlier estimates regarding its toxicity might be overrated, [17]. Besides increasing oil content and altering fatty acid composition, breeders are also focussing on improving yield, growth and tolerance to biotic and abiotic stress, including tolerance to plant pathogens and herbicides (see Sect. “Overview of market-oriented NGT applications in Brassicaceae oilseed plants” and references in Tables 1, 2 and S1-S3). Changes in these traits often result in positive fitness effects like higher likelihood of persistence and spread (see Sect. “Overview of market-oriented NGT applications in Brassicaceae oilseed plants”).
The genomes of some Brassicaceae species are particularly complex, which means that conventional breeding (including non-targeted mutagenesis) is not only time- and labour-intensive for many traits, but also faces practical limitations [18, 19]. Several Brassicaceae crops are polyploid (up to allohexaploid), meaning they have more than two sets of paired chromosomes and therefore more than two copies of each gene. In addition, all Brassica species have undergone genome triplication, which has resulted in a high number of gene copies [18, 20]. In order to achieve a desired breeding characteristic, it is frequently necessary to knock out all homologous genes [21], and in certain cases this may not be practicable at all.
New genomic techniques (NGTs), such as CRISPR/Cas9, have been successfully applied in Brassicaceae [18, 22, 23]. The technology has made it possible to alter multiple copies of a gene, or even alter several different genes simultaneously (multiplexing). Unlike conventional breeding, NGTs can be used to introduce changes in genomic regions, in which natural mutations are known to occur at a low rate [24]. Due to the plants’ genome organisation, these regions are less accessible to conventional breeding methods, including non-targeted mutagenesis, than other genomic regions [25, 26]. This technical potential of NGTs allows genotypes and traits to be modified within short periods of time, also going beyond what can be expected from conventional breeding [27,28,29,30].
While some of the Brassicaceae species developed with the means of NGTs were also achieved using conventional breeding (e.g. EMS (ethyl methane sulphonate) breeding), others are novel [31]. In future, it is expected that different NGT-derived genotypes can be generated [32,33,34,35], combined and stacked in plant breeding [36,37,38,39], thus leading to even more extensive overall genomic and phenotypic changes in NGT plants.
Besides scientific publications dealing with basic and/or applied research on NGT Brassicaceae (hereinafter referred to as ‘NGT applications’), which will be evaluated as part of this study, various projects are already at a more or less advanced stage of commercialisation and have been tested in field trials (see Tables 1 and 2). For example, in 2024, the company Bayer announced to bring CRISPR mustard plants (B. juncea) to the market. These plants are the first commercially available Brassicaceae NGT worldwide and were designed to have less pungent-tasting leaves [40].
The following sections provide an overview of current NGT applications in several Brassicaceae oilseed crops, i.e. oilseed rape (B. napus), camelina (C. sativa) and pennycress (T. arvense). In addition, the biology of Brassicaceae oilseed crops is introduced, potential hazards are identified and specific risk scenarios are developed for some NGT applications. For the purpose of this review, market-oriented NGT applications in Brassicaceae oil plants were identified in the scientific literature and all NGT plants without permanent insertion of transgenes were listed. Basic research projects and applications using oligonucleotide directed mutagenesis (ODM) methods were not considered.
Biology of relevant Brassicaceae oilseed crops
The biology of Brassica napus
Oilseed rape is allotetraploid (2n = 38, AACC) [41] and the result of a natural cross between the diploid Brassica species B. oleracea (2n = 18, CC) and B. rapa (2n = 20, AA). The evolutionary relationship between different Brassica species sharing three core genomes (A, B and C) is described in the ‘Triangle of U’ [42]. Europe is the centre of origin and genetic diversity for oilseed rape and several other Brassica species. Due to its origin, B. napus is well adapted to European climatic conditions. However, oilseed rape is grown globally and is one of the most important oilseed crops in economic terms [43]. Canola oil, derived from double-zero oilseed rape seeds with low erucic acid and glucosinolate levels, is a healthy and nutritionally balanced cooking oil. It is also an important source of biodiesel production, industrial oil and proteins for animal feed [43].
Weedy characteristics
Most cultivated Brassica species have certain weedy characteristics, e.g. increased dispersal abilities, long secondary seed dormancy, the ability to invade disturbed areas and natural plant communities. Additionally, several relatives of oilseed rape, e.g. Sinapis arvensis, Raphanus raphanistrum and Hirschfeldia incana, are regarded as weeds. Weedy forms of B. napus, B. rapa and B. oleracea also exist, as oilseed rape has some wild (or weedy) plant characteristics, such as seed shedding and long secondary dormancy of the seeds in the soil. Although weedy oilseed rape is predominantly found on arable land, ruderal (feral) populations can persist over longer periods of time [44,45,46] and they have been found, for example, in various European countries, stemming from imports, transports and other sources (see, amongst others, [47,48,49,50]).
Interspecific hybridisation
Oilseed rape has pronounced hybridisation potential and can form hybrids with various congeneric (i.e. Brassica) species, such as B. rapa (turnip rape, field mustard, birdseed rape, etc.), B. juncea (various mustard varieties) and others, but also with wild and weedy Brassicaceae from other genera [51, 52]. So far, spontaneous hybridisation was confirmed in a total of eight native or cultivated species in Europe [4, 53,54,55,56,57,58].
Pollen flow
Oilseed rape is self-compatible and predominantly self-pollinated, but also cross-pollinated by wind and insects. Estimates of the outcrossing rate vary widely in the literature, ranging from 12 to 55 percent [59, 60]. It can thus be inferred that up to half of the pollen load may be dispersed by wind and, above all, by insects. Cross-pollination over very long distances has been demonstrated in oilseed rape (several kilometres) [61, 62]. It is also a sought-after source of food, especially for bees and outcrossing can be expected in the foraging area of both social and solitary bees [63].
Secondary dormancy
A considerable number of seeds are lost when oilseed rape is harvested, and end up back on the fields as volunteer rapeseed. According to Gulden et al. [64], this loss can be up to around 6% of the harvest, which can amount to a total of 6000 seeds per square metre [65]. Around 80% of volunteers germinate during the first 12 months after harvest, but a considerable proportion of the seeds remain viable for up to 15 years in the soil [66,67,68,69].
The biology of Camelina sativa
Camelina (C. sativa) is an annual Brassicaceae plant that is mainly cultivated in Europe and North America. It is allohexaploid (2n = 6x = 40, AABBCC) and an ancient crop that has been used i.a. for lamp oil and medical purposes [8]. A recent study found that the plant was probably first cultivated in the Caucasus region [70]. In contrast to other Brassicaceae species, camelina has not been widely used in plant breeding in the past, so only a small number of varieties are available for agricultural purposes [71]. The oil from camelina seeds is used as food and feed, but also for biofuels and industrial compounds [71,72,73]. It contains large amounts of polyunsaturated fatty acids (PUFAs) such as essential linoleic and linolenic acids [73, 74].
Pollen flow
Camelina is primarily self-pollinating, but there is also evidence of cross-pollination by bees, bumblebees and other insects [75]. Outcrossing in camelina decreases rapidly with distance. For example Walsh et al. [76] demonstrated that the outcrossing rate in camelina populations was only 0.09% at a distance of 20 m. However, this does not eliminate the possibility of rare but ecologically significant long-distance pollination events by insects that are able to fly considerable distances [63].
Dormancy
Seed shattering and the small size of camelina seeds (seed rain from the combine harvester) both contribute to harvest losses. Thus, due to primary or secondary dormancy the likelihood of volunteer plants and persistence may increase. There are differing accounts in the literature with regard to secondary dormancy, i.e. dormancy after harvest or seed dispersal. While Ehrensing and Guy [77] found no dormancy in camelina seeds at all, Walsh et al. [78] showed that volunteer populations could be found up to two years after cultivation, even when selective herbicides are used, thus suggesting survival in the seed bank. Similarly another experiment conducted in south-eastern France found that 85.8% of wild camelina seeds at a depth of 10 cm had survived after two and a half years [79]. Thus, while dormancy has been principally documented, the longevity of camelina seeds in the seed bank still needs to be further investigated.
Hybridisation potential
Camelina can hybridise with wild relatives such as C. microcarpa and C. alyssum [80,81,82], which are also part of the European flora. According to results of a large number of studies, interspecific hybridisation with other Brassicaceae such as oilseed rape, field mustard, Arabidopsis and others, seems unlikely (see overview in ref. [75]). However, most of these studies were conducted in North America while data from Europe are largely missing.
The biology of Thlaspi arvense
Pennycress (T. arvense) is another member of the Brassicaceae family and currently being engineered with NGTs. It is closely related to the thale cress Arabidopsis thaliana and native to Eurasia, but can now be found worldwide as crop and weed [83]. Uses include feed and the production of agrofuels [84]. Due to its high vitamin C content, it is also added to salads or is cooked like spinach [85]. Pennycress is diploid, the plant’s genome consists of seven chromosomes (2n = 14) [86]. It has a high seed oil content and a favourable fatty acid composition that makes it suitable for use as oil crop for industrial purposes [87]. In agriculture, pennycress is mainly used as winter cover crop since it can tolerate very low temperatures [86, 88]. As a cover crop, it provides important ecosystem services by reducing soil erosion and the leaching of nutrients.
Weedy characteristics
Pennycress has only recently been domesticated and current cultivars still harbour many weed traits [86]. In North America, it is listed in several regions as a noxious weed which considerably reduces crop yields [85]. It is also considered as a weed in the UK [89] and other European countries [90].
Pollen flow
Pennycress is mainly wind-, but also self- or insect pollinated. In a study conducted in Germany, flowers were visited by different bee species, but not by honey bees [91].
Seed dormancy
Due to its nearly non-domesticated history, T. arvense still exhibits weedy characteristics, for example very long secondary seed dormancy. The seed bank can last 20 years or more [92].
Interspecific hybrids
Interspecific hybridisation is not reported for T. arvense [87].
Pollinator interaction
Pennycress has a high feed value for pollinating insects, and is especially valuable as early-season food source [93].
Overview of market-oriented NGT applications in Brassicaceae oilseed plants
Brassica napus (oilseed rape)
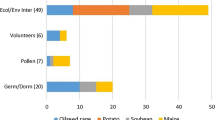
So far, around 45 market-oriented NGT applications in B. napus are described in the scientific literature (see Table S1). The agronomic importance of this oilseed crop has produced a growing interest in exploiting the potential of NGTs to tailor their fatty acid composition and total oil content to specific uses (14 reported NGT applications, shown in orange in Table S1 and Fig. 1). Attempts to reduce PUFAs and erucic acid by knocking out FAD (fatty acid dehydrogenase) and FAE (fatty acid elongase) genes are particularly frequent (see for example [94]). Further alterations in this direction were achieved i.a. by knocking out other genes in the fatty acid biosynthesis (SAD2 (Δ9-Stearoyl-ACP-Desaturase 2) [95]) and lipid decomposition genes (SFAR4 and 5 genes (seed fatty acid reducer 4 and 5) [96]). Alterations of genes in flavonoid pathway (TT genes (transparent testa) [13, 97, 98]) or glucose flux and ABA hormone signalling (CIPK9 gene (Calcineurin B-like (CBL)-interacting protein kinase 9) [99]) also showed strong influence on oil content or fatty acid composition. A CRISPR library was used in a recent study to knock out different genes, partially of unknown function in B. napus, which also significantly altered oil content and fatty acid composition [33].
Three main categories of NGT applications in Brassicaceae oilseed plants B. napus, C. sativa and T. arvense. A total of 58 NGT applications were found in the three species in the scientific literature (see Table S1-3). Attempts to change the oil content or composition are marked in orange. NGT applications that involved traits which are known to be related to fitness effects (and potentially resulting in higher likelihood of persistence and spread) in the plant are marked in green. Other traits are marked in grey
In addition to various applications that intend to improve breeding and harvesting (see for example [100, 101]), there are numerous studies that involve traits that are related to the fitness of plants. Related traits were altered by knocking out diverse genes (see Table S1) and the resulting NGT oilseed crops showed for example increased drought tolerance [102], superior growth [103], longer roots [104], longer flowering period [105] or a higher number of siliques and seeds [106] (green in Table S1 and Fig. 1).
Camelina sativa (camelina)
Camelina has already been genetically engineered several times using NGTs (10 reported NGT applications, see Table S2). Similar to oilseed rape, attempts are being made to alter the oil content and fatty acid composition (8 reported NGT applications, orange in Table S2 and Fig. 1) and NGT applications to reduce PUFAs and erucic acid (by modification of FAD and FAE genes) are particularly frequent as well (see for example [19, 107]). Different knockouts (5 genes, multiple copies) were established in a multiplexing approach resulting in early-flowering plants [32] (green in Table S2 and Fig. 1).
Thlaspi arvense (pennycress)
In pennycress, NGTs were used to knock out FAE1 (see for example [16]), thus making it possible to abolish erucic acid production (see Table S3 and Fig. 1). The knockout strain was further combined with NGT-derived FAD2 and ROD1 knockouts to increase oleic acid content and to decrease PUFA content [108].
Field trials and commercialisation pipeline
In the USA and also in Europe, various projects are underway aiming at the market release of NGT Brassicaceae. In Europe, there is currently no NGT product on the market. However, applications for field trials were filed in both the EU and the UK [109]. All trials were carried out with plants modified with the CRISPR/Cas9 gene scissors (Table 1). After leaving the EU, the UK introduced a new law that facilitate the field release of NGT plants under simplified conditions. Current field trials in the EU and the UK include NGT oilseed rape as well as NGT camelina and NGT cabbage (B. oleracea).
In the US, companies and research institutions can submit a request to the US Department of Agriculture (USDA) to determine whether a transgenic or NGT plant is subject to the requirements of the US “Coordinated Framework for the Regulation of Biotechnology” or may be marketed without restrictions. In the meantime, a number of NGT Brassicaceae have been exempted from biotechnology regulations this way (see Table 2). However, deregulation does not automatically mean that an NGT plant will actually be commercialised. So far, only an NGT brown mustard (B. juncea) developed by Pairwise has been announced to be commercialised by company Bayer. In addition to oilseed rape, some NGT applications in camelina and pennycress have been deregulated, most of which have had their oil content modified. In nearly half of the applications for deregulation, all relevant information was declared to be confidential business information (CBI), thus no information is publicly available concerning the specifics of these projects (Table 2).
Findings and scenarios relevant for risk assessment
This section is not a risk assessment per se and does not derive to final conclusions in regard to specific risks. At the stage of hazard identification, findings from laboratory studies can be highly relevant to develop risk hypothesis which then can be used in further risk assessment. Therefore, this review accomplished a comprehensive overview on the existing information of various scientific quality including some grey literature. Further data and expert judgements will then be required to proof, dismiss or assess specific risks of specific plant/trait combinations.
Hazard identification and risks for pollinators
Brassicaceae oilseed crops are highly attractive for pollinators: they offer easily accessible and highly abundant floral resources and are an important food resource [93, 113]. Their pollen has low protein:lipid values [114] and is particularly rich in PUFA linolenic acid [115].
It has been shown that fatty acids play key roles in the development, communication, reproduction and health of pollinators, including in their colonies (see, e.g. [116,117,118]). Studies indicate that the specific composition of fatty acids in pollen is important for plant choice and pollinator–flower interactions [114]. In addition, it is assumed that the bumblebee species Bombus terrestris relies on lipids as nutritional cue to regulate its food intake. This is likely to be caused by the impact of certain fatty acids on their overall fitness [119].
Changes in the dietary composition of pollinators should be carefully examined for risks to the insects, as a suboptimal nutrient balance is thought to be one of the threats to their populations [120].
Decreased amounts of PUFA may negatively affect the health of pollinators
Several NGT applications in Brassicaceae oilseed crops focus on fatty acid composition in efforts to obtain plants with a low PUFA content. Like other fatty acids, PUFAs have different important functions in many biological processes, both in plants and animals. Animals cannot synthesise PUFAs themselves so they need to be part of their diet [117, 121].
The PUFA content of seeds is mainly reduced by knocking out FAD genes using NGTs [15, 19, 94, 108, 122,123,124,125]. In another experiment, the PUFA content was successfully decreased by knocking out CIPK9 genes [99]. In B. napus it was shown that FAD genes are highly expressed in pollen, thus explaining the high levels of PUFAs [126]. Although it remains to be determined, it can therefore be assumed that the knockout of FAD genes will significantly decrease the PUFA content in pollen.
In habitats with agricultural monocultures of plants with a low PUFA content, bees may suffer from PUFA deficiency when feeding on a limited variety of modified pollen [116, 117]. It was shown that honey bees with a linolenic acid dietary deficiency showed poor performance in both olfactory and tactile associative learning assays [116, 127]. According to preliminary data hypopharyngeal glands in the bees were also smaller [116]. Experimental feeding studies showed that total lipid concentration and the linoleic:linolenic acid ratio are further factors affecting nursing activity [128], brood development [117] and adult longevity in honey bees [117, 129]. Taken together, a balanced PUFA diet is necessary for maintaining proper colony development [117, 128, 129].
Further, PUFAs appear to influence the bacterial gut microbiome of certain bees [130, 131] and in case of honey bees they also inhibit the growth of pathogens, e.g. Paenibacillus larvae and fungal diseases [115, 132, 133]. PUFAs also act as pheromones, e.g. in recognising and discriminating between nestmates and non-nestmates [134], or in attracting further worker bees from their hive [135].
Taken together, a sufficient supply of PUFAs appears to be very important for the health, colony development and communication of honey bees. Malnutrition resulting from dietary deficiency of certain fatty acids may therefore cause adverse effects especially in honey bees and maybe also in other wild pollinators, and should be addressed in hazard identification and risk assessment before NGT Brassicaceae with an altered fatty acid content are placed on the market.
Increasing amounts of oil and unsaturated fatty acids may negatively affect the health of pollinators
In addition to approaches aiming to lower the PUFA content in oilseed crops, there are several studies that used NGTs successfully to increase the total amount of oil and, in particular, unsaturated fatty acids in seeds.
For example, oleic acid contents were elevated by knocking out FAD genes [15, 19, 94, 108, 122,123,124,125]. PUFA contents were elevated by knocking out different TT (transparent testa) genes [13, 97, 98], SAD2 (Δ9-Stearoyl-ACP-Desaturase 2) gene [95], KANT3 gene [33], GIF1 (GRF-interacting factor 1) gene [33], AGP11 (Arabinogalactan protein 11) gene [33] or EDA32 gene [33]. In some cases, complex biosynthetic pathways (flavonoid biosynthesis, TT genes) were modified and PUFAs levels increased as (unintended) side effect [13, 97, 98]. In Brassicaceae, such knockouts could also change the fatty acid composition of its pollen and thus influence pollinators' diets.
Studies have shown that the reproduction and survival rate of bumble bees was significantly reduced when they animals ingested too many fatty acids [119, 136]. This may explain why bumble bees strongly avoided consuming pollen enriched with fatty acids in feeding experiments [119]. In honey bees, feeding studies found significant increases in the mortality rate when a certain threshold of oleic or linoleic acid was reached [137].
The negative effects of fatty acids on survival and reproduction could be the result of intoxication with excessive amounts of fatty acids [119]. Intake of high amounts of PUFAs, for example, might lead to lipid peroxidation and cell damage [136]. On the other hand, a lack of other nutrients as a consequence of reduced overall pollen consumption due to fat avoidance, might further increase mortality [119]. Hence, in habitats with agricultural monocultures of plants with highly increased oil and PUFA content in pollen, pollinators, such as honey bees and bumble bees, may either over-consume fatty acids or suffer nutrient deficiencies due to fat avoidance.
As mentioned above, changes in oil composition of plants can also be achieved with conventional breeding (including random mutagenesis (see also ref. [138]). However, NGTs offer new potential for the targeted editing of Brassicaceae genomes [33]. With regard to individual NGT traits, it may be difficult without an in-depth case-by-case investigation to conclude on whether a specific genotype and phenotype could also be obtained with conventional breeding.
Furthermore, the overall number of NGT applications in Brassicaceae oilseed plants and the technological potential of NGTs [25, 34, 35] show a new dimension in the scale (in terms of space and time) of potential releases of plants [139] with novel genotypes and phenotypes [29, 31, 33]. This will complicate any prediction about the potential impacts of NGT plants, especially when compared with conventionally bred plants: both spontaneous and intended crossings may lead to new combinations of traits causing unintended interactions without precedent (see sect. “Persistence, spread and gene flow”.).
Further observations regarding changes in oil composition
Changed oil content and composition may result in further unintended and undesirable effects. Fatty acids are known to play important roles in plants, e.g. in the biosynthesis of secondary metabolites found in cellular membranes and phytohormones.
For example, the phytohormone group of jasmonate and its derivatives have important roles as signalling molecules in plant defence, particularly against insect herbivores [140, 141]. Linolenic acid is a precursor molecule of jasmonic acid [142]. Changes in oil composition may therefore affect plant communication, signalling pathways and plant resistance to biotic or abiotic stressors [31]. There are further NGT applications with altered plant characteristics which could be relevant to interactions with pollinators, e.g. traits with a significant impact on hormone signalling [99, 143] or flavonoid content [13, 98]. In addition, changes in oil composition may also have effects on various other species which feed on plants, such as pest insects [144] or other wild species [145].
Persistence, spread and gene flow
In environmental risk assessment of genetically engineered organisms, persistence, spread and gene flow are highly relevant for hazard identification and risk assessment [146]. For example, concerns were raised about unpredictable next-generation effects if transgenic plants ‘go wild’ [147].
Due to the biology of Brassicaceae species, it may not be easy to control the further spread of NGT Brassicaceae beyond the areas of intended cultivation, especially under European conditions. Some of the problems are:
-
A broad range of Brassica species can hybridise with each other;
-
Many Brassicaceae have weedy characteristics;
-
Seeds can exhibit prolonged dormancy;
-
Pollination by insects and wind can occur over long distances.
Oilseed rape is also a good example of the difficulties associated with the cultivation of genetically engineered Brassica plants. Sohn et al. [148] showed that the unintended persistence and spread of (transgenic) genetically engineered (GE) oilseed rape is already present in different countries (Japan, Canada, USA, Switzerland, Argentina). The list includes countries that do not even allow the cultivation or import of transgenic plants, as transgenic populations near ports and along transport routes can originate from transgenic seeds that are imported legally or from contamination of other commodities [149].
GE herbicide-tolerant oilseed rape was recently shown to have hybridised with weedy B. rapa (bird rape mustard) in Canada [150]. Oilseed rape and B. rapa are intercrossable, and hybrids of both were confirmed previously (see [151, 152]). It was assumed that the hybrid plants had reduced fertility and were, therefore, unable to become permanently established in the environment. Contrary to this assumption, Laforest et al. showed that the genetically engineered trait is now detectable in B. rapa plants in Canada [150].
The oilseed rape example shows that genetically engineered Brassicaceae plants can persist in the environment as well as spread into wild or weedy populations by pollen or seed dispersal. Other studies appear to indicate fitness advantages of GE oilseed rape or its hybrids with related species (see examples in ref. [147]).
A closer look at NGT applications in Brassicaceae species shows there are several examples of traits with positive fitness effects (green in Tables S2 and S3). It can be hypothesised that these traits might further increase the likelihood of spread and persistence of NGT Brassicaceae plants. Their potential to spread may dependent on environmental conditions: this has been, for example, discussed for semi-dwarf phenotypes [153, 154], which might show improved survival in ruderal areas [155]. However, this paper does not claim evidence on enhanced fitness.
Spontaneous crossings and stacking
NGTs make it possible to induce small, targeted changes in plant genomes to generate new properties. This is, in particular, the case with site-directed nuclease 1 applications (SDN 1, i.e. short deletions and/or insertions of a few base pairs), which also enable the alteration of several different DNA sequences—and thus several properties—simultaneously (multiplexing).
As shown in the overview of applications used in Brassicaceae oilseed plants, most traits are currently based on the knockout of single genes, gene copies, members of a particular gene family or genes associated with a distinct phenotype (see [18, 31]). The two most prominent trait categories, altered fatty acid metabolism and positive fitness effects (orange and green in Table S1-3), comprise a variety of different traits, ranging from early/late flowering and the reduction of flavonoids to the increase/reduction of unsaturated fatty acids (see Table S1-3).
These traits are frequently influenced by many different genes that are involved in multiple biological processes, consequently their alteration may lead to undesirable pleiotropic effects and unintended consequences in some circumstances. Therefore, the potential negative impacts on ecosystems, food webs and the health of wild species, livestock and humans need to be assessed on a case-by-case basis, individually for each event. In addition, possible combinations of the traits and impacts thereof need to be assessed and monitored in the areas of release and in wider environments. Trait combinations may occur through intentional crossings (stacked event) or in case of Brassicaceae, by spontaneous hybridisation (see for example [156]). Consequently, it is likely that new combinations of geno- and phenotypes of Brassicaceae will emerge that were neither intended nor previously tested for biosafety.
In case of large-scale releases of NGT Brassicaceae oilseed plants, the likelihood of hybridisation within crossable species and therefore the overall likelihood for novel traits in ferals or wild relatives would increase. Furthermore, the chances of spontaneous and unintended combinations of different NGT traits would also increase. Similar outcrossing phenomena have already been reported in the cultivation of transgenic oilseed rape plants where spontaneous combinations of herbicide resistances have been observed. These spontaneous combinations resulted in the occurrence of transgenic events that were not approved for release (see [157, 158]).
The stacking of different NGT events via conventional breeding or spontaneous crossings in the environment can both result in NGT offspring with new biological characteristics that are absent in the parental plants (see [147]). New biological characteristics could, for example, affect plant species composition with unintended and possibly undesirable consequences for ecosystems, food webs, the health of wild species and consumers. They may also cause NGT plants to become invasive. Even if each of the initial NGT events were individually classified as ‘safe’ or ‘unlikely to become invasive’ in risk assessment, their offspring may show effects in the next generations causing unexpected risks induced by genomic interactions. As with transgenic plants, spontaneous crossings of NGT plants may result in new combinations of traits, such as higher fitness and/or novel changes in oil composition.
In this context, unpredictable interactions at the genome as well as the phenotype level may further depend on the genetic background (including cryptic gene variants, see for example [159]) and the accumulation of unintended genetic changes caused by the NGT processes (for overview, see [139, 160, 161]).
Conclusions
A closer look at NGT applications in Brassicaceae oilseed crops described in the scientific literature shows that various, often fitness-relevant traits have been altered so far, e.g. growth and yield, oil and protein composition, abiotic and biotic resistance and fertility.
Currently, most of these traits are conferred by knocking out single genes or gene copies. Some of these traits may also be altered with conventional breeding methods. However, the power and increasing complexity of NGT applications, which includes the modification of several different genes [32], means that it is becoming increasingly difficult if not impossible to achieve the same or ‘equivalent’ results using conventional methods. This is due, in particular, to the complexity of some Brassicaceae genomes, but also to the specific technical potential of NGTs that may rapidly evolve further [33,34,35].
The use of NGTs opens up new possibilities to modify the genome [25, 29, 36,37,38], and in many cases results in new genotypes and phenotypes that are unlikely to result from conventional breeding even if additional DNA sequences are not inserted and no new proteins are produced by the plants [27, 28, 162]. Furthermore, the spatio-temporal scale of potential releases of Brassicaceae NGT oilseed plants may also cause new combinations and interactions to occur that were neither intended nor assessed and going along with unpredictable environmental impacts [139, 147]. However, there is an ongoing discussion on the equivalence of new breeding with conventional breeding technologies (see e.g. [163, 164]).
In case of a changed oil composition an increase or decrease of PUFAs may result in risks for the health and survival of pollinators. The likelihood of outcrossing and spread, and environmental risks would increase depending on a couple of factors: (a) the number of released new genotypes; (b) the spatio-temporal scale of release of new genotypes; (c) the fitness relevance of the new traits and (d) the novelty of traits compared to the wildtype. Persistence and spread of novel genotypes can hardly be controlled and also hazard mitigation will be extremely difficult due to the hybridisation potential, seed dormancy, seed dispersal, persistence in the wild and large pollen transfer distances.
Environmental risk scenarios of specific NGT applications in Brassicaceae oilseed crops as outlined in this review, show that a combination of relevant factors has to be addressed here: (a) the technological specificity (with the potential to develop increasingly complex traits); (b) the plants’ biology and (c) the scale of releases. Therefore, the potential release of NGT plants for agricultural purposes requires risk assessment and monitoring not only of single traits, but also of combinatorial and long-term cumulative effects as already foreseen in Directive 2001/18/EC. In addition, risk management may require new concepts and measures to control and potentially limit the scale of releases. This is especially relevant for potential releases of NGT Brassicaceae in Europe which is one of the centres of origin and diversity of many members of this plant family.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- CBI:
-
Confidential business information
- CRISPR Cas:
-
Clustered regularly interspaced short palindromic repeats/CRISPR associated
- EMS:
-
Ethyl methane sulphonate
- FAD:
-
Fatty acid dehydrogenase
- FAE:
-
Fatty acid elongase
- GE:
-
(Transgenic) genetically engineered
- MUFA:
-
Monounsaturated fatty acids
- NGT(s):
-
New genomic technique(s)
- ODM:
-
Oligonucleotide directed mutagenesis
- PUFA:
-
Polyunsaturated fatty acids
- SDN1:
-
Nuclease 1 applications
- USDA:
-
US Department of Agriculture
References
Dixon GR (2006) Origins and diversity of Brassica and its relatives. In: Dixon GR (ed) Vegetable brassicas and related crucifers. CABI, Wallingford, pp 1–33. https://doi.org/10.1079/9780851993959.0001
Arias T, Beilstein MA, Tang M et al (2014) Diversification times among Brassica (Brassicaceae) crops suggest hybrid formation after 20 million years of divergence. Am J Bot 101:86–91. https://doi.org/10.3732/ajb.1300312
Guo Y, Chen S, Li Z, Cowling WA (2014) Center of origin and centers of diversity in an ancient crop, Brassica rapa (Turnip Rape). J Hered 105:555–565. https://doi.org/10.1093/jhered/esu021
FitzJohn RG, Armstrong TT, Newstrom-Lloyd LE et al (2007) Hybridisation within Brassica and allied genera: evaluation of potential for transgene escape. Euphytica 158:209–230. https://doi.org/10.1007/s10681-007-9444-0
Ellstrand NC, Meirmans P, Rong J et al (2013) Introgression of crop alleles into wild or weedy populations. Annu Rev Ecol Evol Syst 44:325–345. https://doi.org/10.1146/annurev-ecolsys-110512-135840
Downey RK (1966) Breeding for fatty acid composition in oils of Brassica napus L. and B. campestris L. Plant Food Hum Nutr 13:171–180. https://doi.org/10.1007/BF01103401
Möllers C, Schierholt A (2002) Genetic variation of palmitate and oil content in a winter oilseed rape doubled haploid population segregating for oleate content. Crop Sci 42:379–384. https://doi.org/10.2135/cropsci2002.3790
Zanetti F, Alberghini B, Marjanović Jeromela A et al (2021) Camelina, an ancient oilseed crop actively contributing to the rural renaissance in Europe: a review. Agron Sustain Dev 41:2. https://doi.org/10.1007/s13593-020-00663-y
Sharafi Y, Majidi MM, Goli SAH, Rashidi F (2015) Oil content and fatty acids composition in Brassica species. Int J Food Prop 18:2145–2154. https://doi.org/10.1080/10942912.2014.968284
Rodríguez-Rodríguez MF, Moreno-Pérez AJ, Makni S et al (2021) Lipid profiling and oil properties of Camelina sativa seeds engineered to enhance the production of saturated and omega-7 fatty acids. Ind Crops Prod 170:113765. https://doi.org/10.1016/j.indcrop.2021.113765
Abedi E, Sahari MA (2014) Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci Nutr 2:443–463. https://doi.org/10.1002/fsn3.121
Kamal-Eldin A (2006) Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur J Lipid Sci Technol 108:1051–1061. https://doi.org/10.1002/ejlt.200600090
Xie T, Chen X, Guo T et al (2020) Targeted knockout of BnTT2 homologues for yellow-seeded Brassica napus with reduced flavonoids and improved fatty acid composition. J Agric Food Chem 68:5676–5690. https://doi.org/10.1021/acs.jafc.0c01126
Knothe G (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuels 22:1358–1364. https://doi.org/10.1021/ef700639e
Okuzaki A, Ogawa T, Koizuka C et al (2018) CRISPR/Cas9-mediated genome editing of the fatty acid desaturase 2 gene in Brassica napus. Plant Physiol Biochem 131:63–69. https://doi.org/10.1016/j.plaphy.2018.04.025
Esfahanian M, Nazarenus TJ, Freund MM et al (2021) Generating pennycress (Thlaspi arvense) seed triacylglycerols and acetyl-triacylglycerols containing medium-chain fatty acids. Front Energy Res 9:620118. https://doi.org/10.3389/fenrg.2021.620118
Galanty A, Grudzińska M, Paździora W, Paśko P (2023) Erucic acid - both sides of the story: a concise review on its beneficial and toxic properties. Molecules 28:1924. https://doi.org/10.3390/molecules28041924
Li J, Yu X, Zhang C et al (2022) The application of CRISPR/Cas technologies to Brassica crops: current progress and future perspectives. aBIOTECH 3:146–161. https://doi.org/10.1007/s42994-022-00076-3
Morineau C, Bellec Y, Tellier F et al (2017) Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa. Plant Biotechnol J 15:729–739. https://doi.org/10.1111/pbi.12671
Wang X, Wang H, Wang J et al (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43:1035–1039. https://doi.org/10.1038/ng.919
Wells R, Trick M, Soumpourou E et al (2014) The control of seed oil polyunsaturate content in the polyploid crop species Brassica napus. Mol Breeding 33:349–362. https://doi.org/10.1007/s11032-013-9954-5
Tian Q, Li B, Feng Y et al (2022) Application of CRISPR/Cas9 in rapeseed for gene function research and genetic improvement. Agronomy 12:824. https://doi.org/10.3390/agronomy12040824
Ahmad N, Fatima S, Mehmood MA et al (2023) Targeted genome editing in polyploids: lessons from Brassica. Front Plant Sci 14:1152468. https://doi.org/10.3389/fpls.2023.1152468
Monroe JG, Srikant T, Carbonell-Bejerano P et al (2022) Mutation bias reflects natural selection in Arabidopsis thaliana. Nature 602:101–105. https://doi.org/10.1038/s41586-021-04269-6
Kawall K (2019) New possibilities on the horizon: genome editing makes the whole genome accessible for changes. Front Plant Sci 10:525. https://doi.org/10.3389/fpls.2019.00525
Eckerstorfer MF, Dolezel M, Engelhard M et al (2023) Recommendations for the assessment of potential environmental effects of genome-editing applications in plants in the EU. Plants 12:1764. https://doi.org/10.3390/plants12091764
ANSES (2023) AVIS de l’Anses relatif à l’analyse scientifique de l’annexe I de la proposition de règlement de la Commission européenne du 5 juillet 2023 relative aux nouvelles techniques génomiques (NTG)—Examen des critères d’équivalence proposés pour définir les plantes NTG de catégorie 1. In: Anses-Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail. https://www.anses.fr/fr/content/avis-2023-auto-0189. Accessed 2 Feb 2024
Eckerstorfer M, Heissenberger A (2023) New Genetic Engineering—possible unintended effects. Verlag Arbeiterkammer Wien
Kawall K (2021) The generic risks and the potential of SDN-1 applications in crop plants. Plants 10:2259. https://doi.org/10.3390/plants10112259
Bohle F, Schneider R, Mundorf J et al (2024) Where does the EU-path on new genomic techniques lead us? Front Genome Ed 6:1377117. https://doi.org/10.3389/fgeed.2024.1377117
Kawall K (2021) Genome-edited Camelina sativa with a unique fatty acid content and its potential impact on ecosystems. Environ Sci Eur 33:38. https://doi.org/10.1186/s12302-021-00482-2
Bellec Y, Guyon-Debast A, François T et al (2022) New flowering and architecture traits mediated by multiplex CRISPR-Cas9 gene editing in hexaploid Camelina sativa. Agronomy 12:1873. https://doi.org/10.3390/agronomy12081873
He J, Zhang K, Yan S et al (2023) Genome-scale targeted mutagenesis in Brassica napus using a pooled CRISPR library. Genome Res 33:798–809. https://doi.org/10.1101/gr.277650.123
Rönspies M, Schindele P, Puchta H (2021) CRISPR/Cas-mediated chromosome engineering: opening up a new avenue for plant breeding. J Exp Bot 72:177–183. https://doi.org/10.1093/jxb/eraa463
Li J, Li Y, Wang R et al (2022) Multiple functions of MiRNAs in Brassica napus L. Life 12:1811. https://doi.org/10.3390/life12111811
Zetsche B, Heidenreich M, Mohanraju P et al (2017) Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat Biotechnol 35:31–34. https://doi.org/10.1038/nbt.3737
Kawall K, Cotter J, Then C (2020) Broadening the GMO risk assessment in the EU for genome editing technologies in agriculture. Environ Sci Eur 32:106. https://doi.org/10.1186/s12302-020-00361-2
Raitskin O, Patron NJ (2016) Multi-gene engineering in plants with RNA-guided Cas9 nuclease. Curr Opin Biotechnol 37:69–75. https://doi.org/10.1016/j.copbio.2015.11.008
Ma C, Zhu C, Zheng M et al (2019) CRISPR/Cas9-mediated multiple gene editing in Brassica oleracea var. capitata using the endogenous tRNA-processing system. Hortic Res 6:20. https://doi.org/10.1038/s41438-018-0107-1
Karlson D, Mojica JP, Poorten TJ et al (2022) Targeted mutagenesis of the multicopy myrosinase gene family in allotetraploid Brassica juncea reduces pungency in fresh leaves across environments. Plants 11:2494. https://doi.org/10.3390/plants11192494
An H, Qi X, Gaynor ML et al (2019) Transcriptome and organellar sequencing highlights the complex origin and diversification of allotetraploid Brassica napus. Nat Commun 10:2878. https://doi.org/10.1038/s41467-019-10757-1
Nagaharu U (1935) Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn J Bot 7:389–452
Hu D, Jing J, Snowdon RJ et al (2021) Exploring the gene pool of Brassica napus by genomics-based approaches. Plant Biotechnol J 19:1693–1712. https://doi.org/10.1111/pbi.13636
Pascher K, Macalka S, Rau D et al (2010) Molecular differentiation of commercial varieties and feral populations of oilseed rape (Brassica napus L.). BMC Evol Biol 10:63. https://doi.org/10.1186/1471-2148-10-63
Pascher K, Hainz-Renetzeder C, Gollmann G, Schneeweiss GM (2017) Spillage of viable seeds of oilseed rape along transportation routes: ecological risk assessment and perspectives on management efforts. Front Ecol Evol 5:104. https://doi.org/10.3389/fevo.2017.00104
Banks G (2014) Feral oilseed rape populations within a Scottish landscape: implications for GM coexistence and environmental risk assessment. Doctoral dissertation, University of Dundee
SCRI (2004) SIGMEA sustainable introduction of GM crops into European Agriculture. https://cordis.europa.eu/docs/results/501/501986/126792601-6_en.pdf
Squire GR, Breckling B, Dietz Pfeilstetter A et al (2011) Status of feral oilseed rape in Europe: its minor role as a GM impurity and its potential as a reservoir of transgene persistence. Environ Sci Pollut Res 18:111–115. https://doi.org/10.1007/s11356-010-0376-1
Frieß JL, Breckling B, Pascher K, Schröder W (2020) Case study 2: oilseed rape (Brassica napus L.). In: von Gleich A, Schröder W (eds) Gene drives at tipping points: precautionary technology assessment and governance of new approaches to genetically modify animal and plant populations. Springer International Publishing, Cham, pp 103–145
Pascher K, Hainz-Renetzeder C, Jagersberger M et al (2023) Contamination of imported kernels by unapproved genome-edited varieties poses a major challenge for monitoring and traceability during transport and handling on a global scale: inferences from a study on feral oilseed rape in Austria. Front Genome Ed 5:1176290. https://doi.org/10.3389/fgeed.2023.1176290
Chèvre AM, Ammitzbøll H, Breckling B et al (2004) A review on interspecific gene flow from oilseed rape to wild relatives. In: den Nijs HCM, Bartsch D, Sweet J (eds) Introgression from genetically modified plants into wild relatives. CABI Publishing, Wallingford, pp 235–251
Pascher K, Narendja F, Rau D (2006) Feral oilseed rape: investigations on its potential for hybridisation; Final Report in Commission of the Federal Ministry of Health and Women (BMGH), Section IV; GZ: 70420/0116-IV/B/12/2004. Bundesministerium für Gesundheit u. Frauen
Theenhaus A, Zeitler R, Von Brackel W et al (2002) Langzeitmonitoring möglicher Auswirkungen gentechnisch veränderter Pflanzen auf Pflanzengesellschaften Konzeptentwicklung am Beispiel von Raps (Brassica napus). UWSF 14:229–236. https://doi.org/10.1065/uwsf2002.09.044
Breckling B, Middelhoff U, Borgmann P, et al (2003) Biologische Risikoforschung zu gentechnisch veränderten Pflanzen in der Landwirtschaft: Das Beispiel Raps in Norddeutschland. In: Gene, Bits und Ökosysteme. Implikationen neuer Technologien für die ökologische Theorie. pp 19–45
Devos Y, De Schrijver A, Reheul D (2009) Quantifying the introgressive hybridisation propensity between transgenic oilseed rape and its wild/weedy relatives. Environ Monit Assess 149:303–322. https://doi.org/10.1007/s10661-008-0204-y
OECD (2012) Consensus document on the biology of the Brassica crops (Brassica spp.). https://www.oecd.org/science/biotrack/27531440.pdf
COGEM (2013) Genetically modified oilseed rape (Brassica napus). Aspects in relation to the environmental risk assessment and post‐market environmental monitoring of import applications. COGEM advisory report (CGM/130402‐01). https://cogem.net/app/uploads/2019/07/130402-01-Advisory-report-Genetically-modified-oilseed-rape.pdf
Marotti I, Whittaker A, Benedettelli S et al (2020) Evaluation of the propensity of interspecific hybridization between oilseed rape (Brassica napus L.) to wild-growing black mustard (Brassica nigra L.) displaying mixoploidy. Plant Sci 296:110493. https://doi.org/10.1016/j.plantsci.2020.110493
Becker HC, Damgaard C, Karlsson B (1992) Environmental variation for outcrossing rate in rapeseed (Brassica napus). Theoret Appl Genet 84:303–306. https://doi.org/10.1007/BF00229487
Darmency H (1997) Gene flow between crops and weeds: risk for new herbicide resistant weeds ? In: De Prado R, Jorrín J, García-Torres L (eds) Weed and crop resistance to herbicides. Springer, Dordrecht, pp 239–248. https://doi.org/10.1007/978-94-011-5538-0_26
Beckie HJ, Warwick SI, Nair H, Séguin-Swartz G (2003) Gene flow in commercial fields of herbicide-resistant canola (Brassica napus). Ecol Appl 13:1276–1294. https://doi.org/10.1890/02-5231
Ramsay G, Thompson C, Squire G (2003) Quantifying landscape-scale gene flow in oilseed rape. Final Report of DEFRA Project RG0216: an experimental and mathematical study of the local and regional scale movement of an oilseed rape transgene. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=c3ac2c46e805ddd3293074648f7ef08179f13b69
Pasquet RS, Peltier A, Hufford MB et al (2008) Long-distance pollen flow assessment through evaluation of pollinator foraging range suggests transgene escape distances. Proc Natl Acad Sci USA 105:13456–13461. https://doi.org/10.1073/pnas.0806040105
Gulden RH, Shirtliffe SJ, Thomas AG (2003) Harvest losses of canola (Brassica napus) cause large seedbank inputs. Weed Sci 51:83–86. https://doi.org/10.1614/0043-1745(2003)051[0083:HLOCBN]2.0.CO;2
Gruber S, Lutman P, Squire G, et al (2007) Using the SIGMEA data base to provide an overview of the persistence of seeds of oilseed rape in the context of the coexistence of GM and conventional crops. In: Proc 3rd Int Conf GMCC. pp 261–262. https://www.researchgate.net/publication/285821551_Using_the_SIGMEA_data_base_to_provide_an_overview_of_the_persistence_of_seeds_of_oilseed_rape_in_the_context_of_the_coexistence_of_GM_and_conventional_crops
Lutman PJW, Freeman SE, Pekrun C (2003) The long-term persistence of seeds of oilseed rape (Brassica napus) in arable fields. J Agric Sci 141:231–240. https://doi.org/10.1017/S0021859603003575
Lutman PJW, Berry K, Payne RW et al (2005) Persistence of seeds from crops of conventional and herbicide tolerant oilseed rape (Brassica napus). Proc R Soc B 272:1909–1915. https://doi.org/10.1098/rspb.2005.3166
D’Hertefeldt T, Jørgensen RB, Pettersson LB (2008) Long-term persistence of GM oilseed rape in the seedbank. Biol Lett 4:314–317. https://doi.org/10.1098/rsbl.2008.0123
Belter A (2016) Long-term monitoring of field trial sites with genetically modified oilseed rape (Brassica napus L.) in Saxony-Anhalt, Germany: fifteen years persistence to date but no spatial dispersion. Genes 7:3. https://doi.org/10.1007/978-1-4614-9329-7_8
Brock JR, Ritchey MM, Olsen KM (2022) Molecular and archaeological evidence on the geographical origin of domestication for Camelina sativa. Am J Bot 109:1177–1190. https://doi.org/10.1002/ajb2.16027
Vollmann J, Eynck C (2015) Camelina as a sustainable oilseed crop: contributions of plant breeding and genetic engineering. Biotechnol J 10:525–535. https://doi.org/10.1002/biot.201400200
Shonnard DR, Williams L, Kalnes TN (2010) Camelina-derived jet fuel and diesel: sustainable advanced biofuels. Environ Prog Sustain Energy 29:382–392. https://doi.org/10.1002/ep.10461
Iskandarov U, Kim HJ, Cahoon EB (2014) Camelina: an emerging oilseed platform for advanced biofuels and bio-based materials. In: McCann MC, Buckeridge MS, Carpita NC (eds) Plants and bioenergy. Springer, New York, pp 131–140
Abramovic H, Abram V (2005) Physico-chemical properties, composition and oxidative stability of Camelina sativa oil. Food Technol Biotechnol 43:63–70
CFIA (2017) The biology of Camelina sativa (L.) Crantz (Camelina). https://inspection.canada.ca/plant-varieties/plants-with-novel-traits/applicants/directive-94-08/biology-documents/camelina-sativa-l-/eng/1330971423348/1330971509470. Accessed 25 Jan 2024
Walsh KD, Hills MJ, Martin SL, Hall LM (2015) Pollen-mediated gene flow in Camelina sativa (L.) Crantz. Crop Sci 55:196–202. https://doi.org/10.2135/cropsci2014.03.0194
Ehrensing DT, Guy SO (2008) Camelina. Corvallis, Or. : Extension Service, Oregon State University. https://ir.library.oregonstate.edu/concern/open_educational_resources/n583xv355
Walsh KD, Raatz LL, Topinka KC, Hall LM (2013) Transient seed bank of camelina contributes to a low weedy propensity in Western Canadian cropping systems. Crop Sci 53:2176–2185. https://doi.org/10.2135/cropsci2013.03.0142
Saatkamp A, Affre L, Dutoit T, Poschlod P (2009) The seed bank longevity index revisited: limited reliability evident from a burial experiment and database analyses. Ann Bot 104:715–724. https://doi.org/10.1093/aob/mcp148
Séguin-Swartz G, Nettleton JA, Sauder C et al (2013) Hybridization between Camelina sativa (L.) Crantz (false flax) and North American camelina species. Plant Breed. https://doi.org/10.1111/pbr.12067
Julié-Galau S, Bellec Y, Faure J-D, Tepfer M (2014) Evaluation of the potential for interspecific hybridization between Camelina sativa and related wild Brassicaceae in anticipation of field trials of GM camelina. Transgenic Res 23:67–74. https://doi.org/10.1007/s11248-013-9722-7
Zhang C-J, Auer C (2020) Hybridization between Camelina sativa (L.) Crantz and common Brassica weeds. Ind Crops Prod 147:112240. https://doi.org/10.1016/j.indcrop.2020.112240
Ma J, Wang H, Zhang Y (2023) Research progress on the development of pennycress (Thlaspi arvense L.) as a new seed oil crop: a review. Front Plant Sci 14:1268085. https://doi.org/10.3389/fpls.2023.1268085
Keadle SB, Sykes VR, Sams CE et al (2023) National winter oilseeds review for potential in the US Mid-South: Pennycress, Canola, and Camelina. J Agron 115:1415–1430. https://doi.org/10.1002/agj2.21317
Mitich LW (1996) Field pennycress (Thlaspi arvense L.)—the stinkweed. Weed Technol 10:675–678. https://doi.org/10.1017/S0890037X00040604
McGinn M, Phippen WB, Chopra R et al (2019) Molecular tools enabling pennycress (Thlaspi arvense) as a model plant and oilseed cash cover crop. Plant Biotechnol J 17:776–788. https://doi.org/10.1111/pbi.13014
Warwick SI, Francis A, Susko DJ (2002) The biology of Canadian weeds. 9. Thlaspi arvense L. (updated). Can J Plant Sci 82:803–823. https://doi.org/10.4141/P01-159
López MV, de la Vega M, Gracia R et al (2021) Agronomic potential of two European pennycress accessions as a winter crop under European Mediterranean conditions. Ind Crops Prod 159:113107. https://doi.org/10.1016/j.indcrop.2020.113107
Stroh PA, Humphrey TA, Burkmar RJ, et al (2023) Thlaspi arvense L. In: BSBI Online Plant Atlas 2020
Holm L, Doll J, Holm E et al (1997) World weeds: natural histories and distribution. Wiley and Sons, Hoboken, p 1129
Groeneveld JH, Klein A-M (2014) Pollination of two oil-producing plant species: Camelina (Camelina sativa L. Crantz) and pennycress (Thlaspi arvense L.) double-cropping in Germany. GCB Bioenergy 6:242–251. https://doi.org/10.1111/gcbb.12122
CABI (2014) Thlaspi arvense (field pennycress)—CABI Compendium. https://doi.org/10.1079/cabicompendium.27595
Eberle CA, Thom MD, Nemec KT et al (2015) Using pennycress, camelina, and canola cash cover crops to provision pollinators. Ind Crops Prod 75:20–25. https://doi.org/10.1016/j.indcrop.2015.06.026
Shi J, Ni X, Huang J et al (2022) CRISPR/Cas9-mediated gene editing of BnFAD2 and BnFAE1 modifies fatty acid profiles in Brassica napus. Genes 13:1681. https://doi.org/10.3390/genes13101681
Huang H, Ahmar S, Samad RA et al (2023) A novel type of Brassica napus with higher stearic acid in seeds developed through genome editing of BnaSAD2 family. Theor Appl Genet 136:187. https://doi.org/10.1007/s00122-023-04414-x
Karunarathna NL, Wang H, Harloff H-J et al (2020) Elevating seed oil content in a polyploid crop by induced mutations in SEED FATTY ACID REDUCER genes. Plant Biotechnol J 18:2251–2266. https://doi.org/10.1111/pbi.13381
Li H, Yu K, Zhang Z et al (2023) Targeted mutagenesis of flavonoid biosynthesis pathway genes reveals functional divergence in seed coat colour, oil content and fatty acid composition in Brassica napus L. Plant Biotechnol J 22:445–459. https://doi.org/10.1111/pbi.14197
Zhai Y, Yu K, Cai S et al (2020) Targeted mutagenesis of BnTT8 homologs controls yellow seed coat development for effective oil production in Brassica napus L. Plant Biotechnol J 18:1153–1168. https://doi.org/10.1111/pbi.13281
Wang N, Tao B, Mai J et al (2023) Kinase CIPK9 integrates glucose and abscisic acid signaling to regulate seed oil metabolism in rapeseed. Plant Physiol 191:1836–1856. https://doi.org/10.1093/plphys/kiac569
Zhong Y, Wang Y, Chen B et al (2022) Establishment of a dmp based maternal haploid induction system for polyploid Brassica napus and Nicotiana tabacum. J Integr Plant Biol 64:1281–1294. https://doi.org/10.1111/jipb.13244
Zhai Y, Cai S, Hu L et al (2019) CRISPR/Cas9-mediated genome editing reveals differences in the contribution of INDEHISCENT homologues to pod shatter resistance in Brassica napus L. Theor Appl Genet 132:2111–2123. https://doi.org/10.1007/s00122-019-03341-0
Linghu B, Song M, Mu J et al (2023) Comprehensive analysis of U-box E3 ubiquitin ligases gene family revealed BnPUB18 and BnPUB19 negatively regulated drought tolerance in Brassica napus. Ind Crops Prod 200:116875. https://doi.org/10.1016/j.indcrop.2023.116875
Zheng M, Zhang L, Tang M et al (2020) Knockout of two BnaMAX1 homologs by CRISPR/Cas9-targeted mutagenesis improves plant architecture and increases yield in rapeseed (Brassica napus L.). Plant Biotechnol J 18:644–654. https://doi.org/10.1111/pbi.13228
Yao J, Bai J, Liu S et al (2022) Editing of a novel Cd uptake-related gene CUP1 contributes to reducing Cd accumulations in Arabidopsis thaliana and Brassica napus. Cells 11:3888. https://doi.org/10.3390/cells11233888
Geng R, Shan Y, Li L et al (2022) CRISPR-mediated BnaIDA editing prevents silique shattering, floral organ abscission, and spreading of Sclerotinia sclerotiorum in Brassica napus. Plant Comm. https://doi.org/10.1016/j.xplc.2022.100452
Yang Y, Zhu K, Li H et al (2018) Precise editing of CLAVATA genes in Brassica napus L. regulates multilocular silique development. Plant Biotechnol J 16:1322–1335. https://doi.org/10.1111/pbi.12872
Han L, Haslam RP, Silvestre S et al (2022) Enhancing the accumulation of eicosapentaenoic acid and docosahexaenoic acid in transgenic Camelina through the CRISPR-Cas9 inactivation of the competing FAE1 pathway. Plant Biotechnol J 20:1444–1446. https://doi.org/10.1111/pbi.13876
Jarvis BA, Romsdahl TB, McGinn MG et al (2021) CRISPR/Cas9-induced fad2 and rod1 mutations stacked with fae1 confer high oleic acid seed oil in pennycress (Thlaspi arvense L.). Front Plant Sci 12:652319. https://doi.org/10.3389/fpls.2021.652319
Napier JA (2021) A field day for gene-edited Brassicas and crop improvement. CRISPR J 4:307–312. https://doi.org/10.1089/crispr.2021.29130.kad
Testbiotech (2023) Field trials of plants derived from new genetic engineering—development in Europe. Testbiotech. https://www.testbiotech.org/en/field-trials-new-ge-plants. Accessed 26 Jan 2024
USDA APHIS (2022) Regulated article letters of inquiry. https://web.archive.org/web/20220901100946/https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/am-i-regulated/Regulated_Article_Letters_of_Inquiry. Accessed 2 Feb 2024
USDA APHIS (2024) Regulatory status review table. https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/regulatory-processes/rsr-table/rsr-table. Accessed 2 Feb 2024
Van Reeth C, Michel N, Bockstaller C, Caro G (2019) Influences of oilseed rape area and aggregation on pollinator abundance and reproductive success of a co-flowering wild plant. Agric Ecosyst Environ 280:35–42. https://doi.org/10.1016/j.agee.2019.04.025
Vaudo AD, Tooker JF, Patch HM et al (2020) Pollen protein: lipid macronutrient ratios may guide broad patterns of bee species floral preferences. Insects 11:132. https://doi.org/10.3390/insects11020132
Manning R (2001) Fatty acids in pollen: a review of their importance for honey bees. Bee World 82:60–75. https://doi.org/10.1080/0005772X.2001.11099504
Arien Y, Dag A, Zarchin S et al (2015) Omega-3 deficiency impairs honey bee learning. Proc Natl Acad Sci USA 112:15761–15766. https://doi.org/10.1073/pnas.1517375112
Arien Y, Dag A, Yona S et al (2020) Effect of diet lipids and omega-6:3 ratio on honey bee brood development, adult survival and body composition. J Insect Physiol 124:104074. https://doi.org/10.1016/j.jinsphys.2020.104074
Muth F, Breslow PR, Masek P, Leonard AS (2018) A pollen fatty acid enhances learning and survival in bumblebees. Behav Ecol 29:1371–1379. https://doi.org/10.1093/beheco/ary111
Ruedenauer FA, Raubenheimer D, Kessner-Beierlein D et al (2020) Best be(e) on low fat: linking nutrient perception, regulation and fitness. Ecol Lett 23:545–554. https://doi.org/10.1111/ele.13454
Vanbergen AJ, The Insect Pollinators Initiative (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Envi 11:251–259. https://doi.org/10.1890/120126
Hulbert AJ, Abbott SK, Hulbert AJ, Abbott SK (2012) Nutritional ecology of essential fatty acids: an evolutionary perspective. Aust J Zool 59:369–379. https://doi.org/10.1071/ZO11064
Jiang WZ, Henry IM, Lynagh PG et al (2017) Significant enhancement of fatty acid composition in seeds of the allohexaploid, Camelina sativa, using CRISPR/Cas9 gene editing. Plant Biotechnol J 15:648–657. https://doi.org/10.1111/pbi.12663
Huang H, Cui T, Zhang L et al (2020) Modifications of fatty acid profile through targeted mutation at BnaFAD2 gene with CRISPR/Cas9-mediated gene editing in Brassica napus. Theor Appl Genet 133:2401–2411. https://doi.org/10.1007/s00122-020-03607-y
Lee K-R, Jeon I, Yu H et al (2021) Increasing monounsaturated fatty acid contents in hexaploid Camelina sativa seed oil by FAD2 gene knockout using CRISPR-Cas9. Front Plant Sci 12:702930. https://doi.org/10.3389/fpls.2021.702930
Liu H, Lin B, Ren Y et al (2022) CRISPR/Cas9-mediated editing of double loci of BnFAD2 increased the seed oleic acid content of rapeseed (Brassica napus L.). Front Plant Sci 13:1034215. https://doi.org/10.3389/fpls.2022.1034215
Piffanelli P, Ross JHE, Murphy DJ (1997) Intra- and extracellular lipid composition and associated gene expression patterns during pollen development in Brassica napus. Plant J 11:549–562. https://doi.org/10.1046/j.1365-313X.1997.11030549.x
Arien Y, Dag A, Shafir S (2018) Omega-6:3 ratio more than absolute lipid level in diet affects associative learning in honey bees. Front Psychol 9:1001. https://doi.org/10.3389/fpsyg.2018.01001
Minahan D, Goren M, Shafir S (2024) Unbalanced dietary omega-6:3 ratio affects onset of nursing and nurse–larvae interactions by honey bees, Apis mellifera. Anim Behav. https://doi.org/10.1016/j.anbehav.2024.05.007
Ma L, Wang Y, Hang X et al (2015) Nutritional effect of alpha-linolenic acid on honey bee colony development (Apis mellifera L.). J Apic Sci 59:63–72. https://doi.org/10.1515/jas-2015-0023
Wang X, Zhong Z, Chen X et al (2021) High-fat diets with differential fatty acids induce obesity and perturb gut microbiota in honey bee. Int J Mol Sci 22:834. https://doi.org/10.3390/ijms22020834
Leonhardt SD, Peters B, Keller A (2022) Do amino and fatty acid profiles of pollen provisions correlate with bacterial microbiomes in the mason bee Osmia bicornis? Philos Trans R Soc Lond B Biol Sci 377:20210171. https://doi.org/10.1098/rstb.2021.0171
Ababouch L, Chaibi A, Busta FF (1992) Inhibition of bacterial spore growth by fatty acids and their sodium salts. J Food Prot 55:980–984. https://doi.org/10.4315/0362-028X-55.12.980
Feldlaufer MF, Knox DA, Lusby WR, Shimanuki H (1993) Antimicrobial activity of fatty acids against Bacillus larvae, the causative agent of American foulbrood disease. Apidologie 24:95–99. https://doi.org/10.1051/apido:19930202
Breed MD (1998) Recognition pheromones of the honey bee. Bioscience 48:463–470. https://doi.org/10.2307/1313244
Keeling CI, Slessor KN, Higo HA, Winston ML (2003) New components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proc Natl Acad Sci USA 100:4486–4491. https://doi.org/10.1073/pnas.0836984100
Vaudo AD, Stabler D, Patch HM et al (2016) Bumble bees regulate their intake of essential protein and lipid pollen macronutrients. J Exp Biol 219:3962–3970. https://doi.org/10.1242/jeb.140772
Manning R, Rutkay A, Eaton L, Dell B (2007) Lipid-enhanced pollen and lipid-reduced flour diets and their effect on the longevity of honey bees (Apis mellifera L.). Aust J Entomol 46:251–257. https://doi.org/10.1111/j.1440-6055.2007.00598.x
Havlickova L, He Z, Berger M et al (2023) Genomics of predictive radiation mutagenesis in oilseed rape: modifying seed oil composition. Plant Biotechnol J. https://doi.org/10.1111/pbi.14220
Koller F, Schulz M, Juhas M et al (2023) The need for assessment of risks arising from interactions between NGT organisms from an EU perspective. Environ Sci Eur 35:27. https://doi.org/10.1186/s12302-023-00734-3
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227. https://doi.org/10.1146/annurev.phyto.43.040204.135923
Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66. https://doi.org/10.1146/annurev.arplant.59.032607.092825
Gfeller A, Dubugnon L, Liechti R, Farmer EE (2010) Jasmonate biochemical pathway. Sci Signal. https://doi.org/10.1126/scisignal.3109cm3
Sun Q, Lin L, Liu D et al (2018) CRISPR/Cas9-mediated multiplex genome editing of the BnWRKY11 and BnWRKY70 genes in Brassica napus L. Int J Mol Sci 19:2716. https://doi.org/10.3390/ijms19092716
Hixson SM, Shukla K, Campbell LG et al (2016) Long-chain omega-3 polyunsaturated fatty acids have developmental effects on the crop pest, the cabbage white butterfly Pieris rapae. PLoS ONE 11:e0152264. https://doi.org/10.1371/journal.pone.0152264
Colombo SM, Campbell LG, Murphy EJ et al (2018) Potential for novel production of omega-3 long-chain fatty acids by genetically engineered oilseed plants to alter terrestrial ecosystem dynamics. Agric Syst 164:31–37. https://doi.org/10.1016/j.agsy.2018.03.004
EFSA (2010) Guidance on the environmental risk assessment of genetically modified plants: EFSA Guidance Document on the ERA of GM plants. EFSA J 8:1879. https://doi.org/10.2903/j.efsa.2010.1879
Bauer-Panskus A, Miyazaki J, Kawall K, Then C (2020) Risk assessment of genetically engineered plants that can persist and propagate in the environment. Environ Sci Eur 32:32. https://doi.org/10.1186/s12302-020-00301-0
Sohn S-I, Pandian S, Oh Y-J et al (2021) A review of the unintentional release of feral genetically modified rapeseed into the environment. Biology 10:1264. https://doi.org/10.3390/biology10121264
Schulze J, Brodmann P, Oehen B, Bagutti C (2015) Low level impurities in imported wheat are a likely source of feral transgenic oilseed rape (Brassica napus L.) in Switzerland. Environ Sci Pollut Res 22:16936–16942. https://doi.org/10.1007/s11356-015-4903-y
Laforest M, Martin S, Soufiane B et al (2022) Distribution and genetic characterization of bird rape mustard (Brassica rapa) populations and analysis of glyphosate resistance introgression. Pest Manag Sci 78:5471–5478. https://doi.org/10.1002/ps.7170
Jorgensen RB, Andersen B (1994) Spontaneous hybridization between oilseed rape (Brassica napus) and weedy B. campestris (Brassicaceae): a risk of growing genetically modified oilseed rape. Am J Bot 81:1620–1626. https://doi.org/10.2307/2445340
Warwick SI, Légère A, Simard M-J, James T (2008) Do escaped transgenes persist in nature? The case of an herbicide resistance transgene in a weedy Brassica rapa population. Mol Ecol 17:1387–1395. https://doi.org/10.1111/j.1365-294X.2007.03567.x
Fan S, Zhang L, Tang M et al (2021) CRISPR/Cas9-targeted mutagenesis of the BnaA03.BP gene confers semi-dwarf and compact architecture to rapeseed (Brassica napus L.). Plant Biotechnol J 19:2383–2385. https://doi.org/10.1111/pbi.13703
Song M, Linghu B, Huang S et al (2022) Genome-wide survey of leucine-rich repeat receptor-like protein kinase genes and CRISPR/Cas9-targeted mutagenesis BnBRI1 in Brassica napus. Front Plant Sci 13:865132. https://doi.org/10.3389/fpls.2022.865132
Reuter H, Menzel G, Pehlke H, Breckling B (2008) Hazard mitigation or mitigation hazard? Environ Sci Pollut Res 15:529–535. https://doi.org/10.1007/s11356-008-0049-5
Zhang C-J, Wang Y, Gao Y et al (2021) High population density of bee pollinators increasing Camelina sativa (L.) Crantz seed yield: implications on the potential risk for insect-mediated gene flow. Ind Crops Prod 172:114001. https://doi.org/10.1016/j.indcrop.2021.114001
Aono M, Wakiyama S, Nagatsu M et al (2006) Detection of feral transgenic oilseed rape with multiple-herbicide resistance in Japan. Environ Biosaf Res 5:77–87. https://doi.org/10.1051/ebr:2006017
Schafer MG, Ross AA, Londo JP et al (2011) The establishment of genetically engineered canola populations in the U.S. PLoS ONE 6:e25736. https://doi.org/10.1371/journal.pone.0025736
Aguirre L, Hendelman A, Hutton SF et al (2023) Idiosyncratic and dose-dependent epistasis drives variation in tomato fruit size. Science 382:315–320. https://doi.org/10.1126/science.adi5222
Koller F, Cieslak M (2023) A perspective from the EU: unintended genetic changes in plants caused by NGT—their relevance for a comprehensive molecular characterisation and risk assessment. Front Bioeng Biotechnol 11:1276226. https://doi.org/10.3389/fbioe.2023.1276226
Chu P, Agapito-Tenfen SZ (2022) Unintended genomic outcomes in current and next generation GM techniques: a systematic review. Plants 11:2997. https://doi.org/10.3390/plants11212997
Bohle F, Schneider R, Mundorf J, et al (2023) Where does the EU-path on NGTs lead us?
EFSA (2022) Criteria for risk assessment of plants produced by targeted mutagenesis, cisgenesis and intragenesis. EFSA J 20:e07618. https://doi.org/10.2903/j.efsa.2022.7618
EFSA (2024) Scientific opinion on the ANSES analysis of Annex I of the EC proposal COM (2023) 411 (EFSA‐Q‐2024‐00178)|EFSA. https://www.efsa.europa.eu/en/efsajournal/pub/8894. Accessed 1 Aug 2024
Acknowledgements
We like to thank the experts of the Bundesamt für Naturschutz (BfN) for giving their feedback.
Funding
This work was supported by the German Federal Agency for Nature Conservation (Funding code: 3522840500).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koller, F., Cieslak, M. & Bauer-Panskus, A. Environmental risk scenarios of specific NGT applications in Brassicaceae oilseed plants. Environ Sci Eur 36, 189 (2024). https://doi.org/10.1186/s12302-024-01009-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-024-01009-1